Electroless Plating
Electroless plating, also called auto catalytic plating/chemical deposition, uses no external power source. The metal that is deposited from the anode, is decreased by a chemical reducing agent, not an electrical current. This process is still consider plating because of the reaction that takes place. The reaction happens on the surface of the cathode, not the entire cathode. Below is a formula that represent the chemical reaction.
Mⁿ⁺ + ne⁻→ M
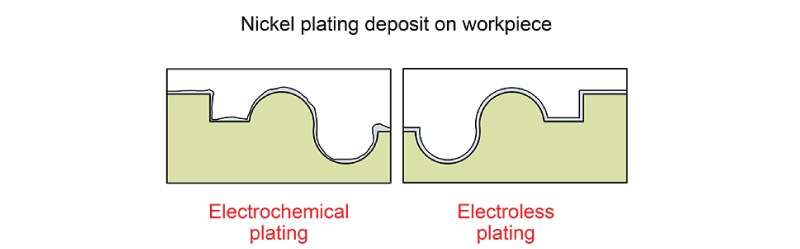
The most common metal that used electroless plating is nickel. Not all metals can be plated using this process. The reducing agents are usually more expensive compared to elector plating with an electrical current. There are numerous benefits to electroless deposition. Electrical deposition can be used to deposit metal on non conductive surfaces. These can include plastics, glass, and ceramics. Some pretreatment steps may need to be used to better activate these surface. Deposits can build up on cathodes in the edges and projections. Auto catalytic plating, reduces hydrogen charging and as unlimited throwing power.
Electroless does has some problems. It can be extremely pricey. One significant problem happens when inadequate surface preparation occurs. The electroless plating is dependent upon adhesion to the surface. A common issue with adhesion is surface contaminates and oils. In order, to avoid corrosion the cathode surface must be coated completely.